Berdasarkan persamaan reaksi (pada T, P) sama: MnO2 + HCl > MnCl2 + H2O + Cl2 maka - Brainly.co.id

Setarakan reaksi redoks berikut menggunakan metode setengah reaksi maupun metode perubahan biloks - Brainly.co.id

How to Balance MnO2 + HCl = Cl2 + MnCl2 + H2O - YouTube

penyetaraan dari reaksi berikut adalah :MnO2+HCl⇒MnCl2 + Cl2 mohon bantuannya.. - Brainly.co.id

Berdasarkan persamaan reaksi (pada t, p) sama: mno2 + hcl > mncl2 + h2o + cl2 maka perbandingan volumenya yaitu . . a. 1, 2, 1,

Mno2+hcl—mncl2+cl2+h2o blance this chemical equation - Brainly.in

Setarakan reaksi redok dgn cara setengah reaksi MnO2 + HCl →MnCl2 + H2O + Cl2 (asam)

PPT - MnO 2 + HCl → MnCl 2 + Cl 2 + H 2 O PowerPoint Presentation, free download - ID:4889625

Cl2 H2o ~ 35+ images how to balance cl2 h2o hcl o2 chlorine gas water, balancear por coeficientes redox mno2 hclmncl2 cl2 h2o, solved provide the major organic product in the reaction

MnO2 + b HCl (c MnCl2 + d H2O + e Cl2Harga koefisien a,b,c,d dan e berturut-turut - Brainly.co.id

Balance the reactions(i) MnO2+HCl—-MnCl2+Cl2+H2O(ii) Fe+H2O—Fe3O4+H2(iii) - Brainly.in

Oxidation Number method. Balance the equation by oxidation Number method. MnO2+HCl=MnCl2+Cl2+H2O - YouTube

Hcl Mno2 ~ 35+ images mno2 hcl mncl2 cl2 h2o, mno2 hcl, mno2 4hclmncl2 2h2o cl2

Koefisien dari mno2(s)+hcl(> mncl2(aq)+h2o(l)+cl2(g)

Pada reaksi redoks mno2 +hcl-> mncl2 +cl2 +h2o yang berperan sebagai reduktor - Brainly.co.id

Solved MnO2 (s) + HCl(aq) => MnCl2(aq) + Cl2(g) + H2O(1) a) | Chegg.com

MnO2 +4HCl MnCl2+2H2O+Cl2 1.tuliskan reaksi oksidasi dan reduksi 2.tentukan oksidator dan - Brainly.co.id

diketahui persamaan reaksi:a MnO2(s) + b HCl(1) = c MnCl2(aq) + d H2O(1) + e Cl2(g) bila sudah di - Brainly.co.id

Solved MnO2(s) + HCl(aq) –> Cl2(g) + MnCl2(aq) + H2O(1) How | Chegg.com
MN O2 ditambah 4 HCL menghasilkan mncl2 + 2 H2O + cl2 tulis zat teroksidasi tereduksi dan oksidator dan reduktor? - Quora

wavut pr alance the following chemical equation. MnO2 + HCL = MnCl2 + Cl2 + H20 HNO3 + Ca(OH)2 = Ca(NO3)2 + H2O Tame a reducing agent which may be used to ob

SOLVED:Balance the following redox equations: (show step by step) KMnO4 + HCl -> KCl + MnCl2 + Cl2 + H2O MnO2 + HCl -> MnCl2 + H2O + Cl2 Cu + HNO3 -> Cu(NO3)2 + NO + H2O

Setarakan persamaan reaksi berikut! KMnO4(aq)+ HCl(aq) → MnCl2(aq) - Mas Dayat

What Is The Oxidation Number Of Mno2

MnO2+HCl->MnCl2+2H2O+Cl2. Perubahan biloks pada unsur mangan adalah A. +2 menjadi +1 B. +2 - Brainly.co.id

wavut pr alance the following chemical equation. MnO2 + HCL = MnCl2 + Cl2 + H20 HNO3 + Ca(OH)2 = Ca(NO3)2 + H2O Tame a reducing agent which may be used to ob

How to balance MnO2+HCl=MnCl2+Cl2+H2O|Chemical equation MnO2+HCl=MnCl2+Cl2+ H2O|MnO2+HCl= - YouTube

diketahui reaksi MnO2 + 4HCl —-MnCl2 + 2H2O + Cl2 tentukan oksidator dan reduktornya - Brainly.co.id

In the following reaction MnO2 + HCl to MnCl2 + H2O class 11 chemistry CBSE
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 is a redox reaction. What are its half-reactions (that shows oxidation and reduction)? - Quora

Chemeketa Community College - ppt download
Perhatikan reaksi berikut : 1. MnO2 + 4 HCl …

balance the chemical equation mno2+Hcl-mncl2+H2o+cl2 - Brainly.in

Half Reactions. - ppt download
Tutorial Menjawab Soal Penyetaraan Reaksi Redoks - Your Chemistry A+

Balance the following chemical equation : Mn02 + HCl MnCl2 + Cl2 + H20

Balance the equation MnO2+HCl=MnCl2+H2O - Science - Chemical Reactions and Equations - 12829559 | Meritnation.com

Balance the following chemical equation : Mn02 + HCl MnCl2 + Cl2 + H20

PPT - Technical Science Introduction to Chemistry PowerPoint Presentation - ID:4347566

Solved 33. Balance the following equations: a. PC13 + H2O → | Chegg.com

In the reaction : MnO_(2) + 4HCI to MnCl_(2) + 2H_(2)O + Cl_(2) (a) Name the compound (i) oxidised, (ii) reduced. (b) Define oxidation and reduction on its basis .

oksidator reduktor reaksi MnO2 + 4HCl - MnCl2 +Cl2 + 2H2O - Brainly.co.id

Chemical Reactions. - ppt download

Balance the chemical equation. kmno4+hcl=kcl+mncl2+h2o+cl2. - YouTube

balance the following chemical equation 1,MnO2 + HCl gives MnCl2 + Cl2 + H2O 2, Fe(S) + H2O(g) - Science - Chemical Reactions and Equations - 11486227 | Meritnation.com

Tentukan reduktor, oksidator, hasil oksidasi dan hasil reduksi dari persamaan reaksi berikut: MNO2 - Brainly.co.id
Solved Consider the reaction: MnO2 + 4HCI –> MnCl2 + Cl2 + | Chegg.com
Soal Kimia X | PDF

MnO2 + HCl → MnCl2 + H2O + Cl2 (Solución): Balance por el Método Algebraico

How to Write the Net Ionic Equation for MnO2 + HCl = Cl2 + MnCl2 + H2O - YouTube

Balance the following chemical reactions a)MnO2 + HCl → MnCl2 + Cl2 + H2O b)Fe (s)+ H2O(g) →Fe3O4 (s) - Brainly.in

Gas klorin dapat dibuat dengan mereaksikan larutan…

Analisis reaksi-reaksi berikut yang termasuk reaksi redoks dan bukan reaksi redoks! a. MnO2 + 4HCl - Mas Dayat

SOLVED:Chlorine forms from the reaction of hydrochloric acid with manganese(IV) oxide. The blanced equation is given below. MnO2 + 4 HCl â†' MnCl2 + Cl2 + 2 H2O Calculate the theoretical yield

Jika pada reaksi; MnO2 + 4HCl → MnCl2 + 2H2O + 2Cl2Dicampurkan 2 mol MnO2 dan 2 mol HCl, maka setelah reaksi berlangsung diperolehA.

Solved 11) Given the equation MnO2(s) + HCl (aq) + Cl2(g) + | Chegg.com
Pada reaksi : MnO2 + 4 HCl àMnCl2 + 2 H2O + Cl2 …

Mno2 + 4 HCL -> MnCL2+2H2O + CL2 reaksi reduktor, oksidator - Brainly.co.id

Solved 1. 2. The laboratory preparation of chlorine is by | Chegg.com
In the reaction : MnO2 + 4HCl→ MnCl2 + Cl2 + 2H2O, the equivalent weight of HCl is:

Ammonia and Chlorine Reaction | NH3 + Cl2 - Traloitructuyen.com
Solved Balancing redox reactions. I need 3-4 of these worked | Chegg.com

Perhatikan reaksi berikut ini! a MnO2 (s) + b HCl (aq) à c MnCl2 (aq) + d Cl2 (g) + e H2O (l) - Brainly.co.id

Setarakan persamaan reaksi dari cucl2(aq)+naoh(> cu(oh)2 (s)+nacl (aq) mno2 (s)+hcl(> mncl2(aq)+h2o(i)+cl2(g)

persamaab reaksi MnO2+4HCL–>MnCl2+2H2O+Cl2 tentunkan senyawa yang sebagai oksidator dan - Brainly.co.id

Pada reaksi : MnO2 + 4HCl -> MnCl2 + 2H2O + Cl…

Redoks dan Elektrokimia - ppt download
![Calculate the volume of Cl2 gas (in ml) liberated at 1 atm & 273 K when 1.74gm MnO, reacts with 2.19gm HCl according to the following reaction with % yield 40. [3]](https://i3.wp.com/toppr-doubts-media.s3.amazonaws.com/images/4730475/1fffa19b-de9b-493c-9721-fc92870992dd.jpg)
Calculate the volume of Cl2 gas (in ml) liberated at 1 atm & 273 K when 1.74gm MnO, reacts with 2.19gm HCl according to the following reaction with % yield 40. [3]

Answered: If 15.6 grams of MnO2 are reacted with… | bartleby

Oxidation - Reduction RedOx. - ppt download

Balance the following equations: (a) H2 + O2 → H2O (b) MnO2 + HCl
Redoks dan Elektrokimia - Tanya MIPI
Balanceo Coef Ind.4to | PDF

A 0 5000 g sample containing MnO2 is treated by concentrated HCl The chlorine gas released from the reaction - Chemistry - Redox Reactions - 12701827 | Meritnation.com

Contoh Soal Penyetaraan Reaksi Redoks Cara PBO suasana basa - YouTube
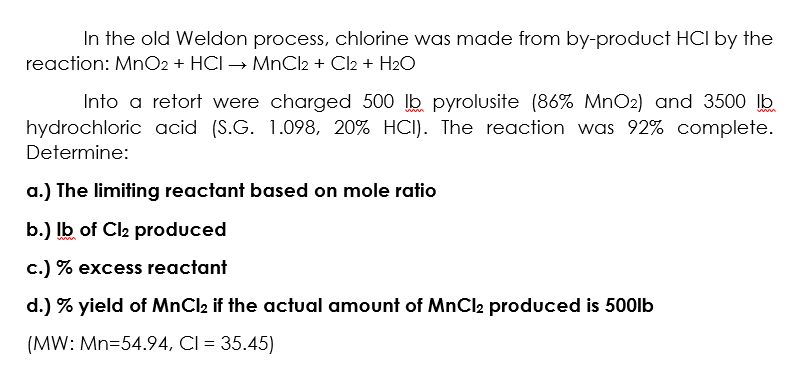
Solved In the old Weldon process, chlorine was made from | Chegg.com

В уравнении HCl + MnO2 → MnCl2 + Cl2 + H2O расставьте коэффициенты методом - Школьные Знания.com

MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 . In the equation, name the compound which is oxidized and which is reduced?
Balance the following equation by oxidation number method. KMnO4 + HCl → KCl + MnCl2 + H2O + Cl2 - Sarthaks eConnect | Largest Online Education Community

Cara Aljabar Penyetaraan Reaksi Presented by : Ibu MF. Endang SL, S.Pd. - ppt download

Setarakan persamaan-persamaan reaksi berikut. a. N2O5(g) + H20(l) → HNO3(aq) b. Ca(OH)2(s) + HBr(aq) → CaBr2(aq) + H2O(l) c.

Balance the following equations: (a) H2 + O2 → H2O (b) MnO2 + HCl

Tentukan agen oksidasi (oksidator) dan agen reduks…

If 1 gm of HCI and 1 gm of MnO2 heated together the maximum ? | Scholr™

Solved 3 Ex: Given the following equation: MnO2 (s) + 4 HCl | Chegg.com

Tarea 1 química 2

write the balanced equation and type of reaction:mno2 +hcl = mncl2 +h2o+cl2 - Brainly.in

by the reactio of 10gm mno2 hcl 224 l cl2 formed calcule composition of mno2 - Chemistry - TopperLearning.com | 6991pn11

Penyetaraan Reaksi Redoks: Cl2 + NaOH → NaCl + NaClO3 + H2O - Urip dot Info

1

Tentukan reaksi oksidasi, reduksi, reduktor dan oksidator dalam persamaan berikut a. cl2 +2ki -> 2kcl + i2 b. mno2 + hcl ->

Mangan(II) klorida - Wikipedia bahasa Indonesia, ensiklopedia bebas

Oxidation-Reduction Topic 9 Review Book. - ppt download

38.) 10 g of MnO2 on reaction with HCl forms 2.24 L of Cl2 (8) at NTP, the percentage impurity of MnO2 is MnO, +4HCI MnCl2 + Cl2 + 2H,0 1) 87% 2) 25% 3) 33.3% 4) 13%

Perhatikan reaksi berikut : PCl3 + Cl2 -> …

Solved What is the sum of the coefficients of the following | Chegg.com

Mno2 + 4hcl = Mncl2 + cl2 + 2h2o which is oxidised and which is r

Tunjukan reaksi oksidasi dan reaksi reduksi dari persamaan reaksi iniA.) MnO2(s) + 4HCl(aq) MnCl2(aq) + Cl2(g) + H2O(1)

Unit 7: Redox & Electrochemistry. - ppt video online download